|
OSOM® Trichomonas Test
SEKISUI DIAGNOSTICS
The only CLIA-waived rapid test for Trichomonas. Detects the antigen so no hurrying to the microscope to see motile organisms. More sensitive than wet mount. Results in 10 minutes or less.
|
|||||||||||||||||||||||||
|
The OSOM® Trichomonas Test is intended for the qualitative detection of Trichomonas vaginalis (“Trichomonas”) antigens from vaginal swabs or from the saline solution prepared when making wet mounts from vaginal swabs. Features and Benefits
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
||||||||||||||||||||||||||
 |
OSOM® Ultra Strep A
SEKISUI DIAGNOSTICS
Clear, accurate results in minutes can help everyone feel better before the office visit is over.VERY ACCURATE – 96% sensitivity; 98% specificity.FAST- Results in 5 minutes.EASY-TO-READ – Two-color results.CONVENIENT – Compact packaging. Available in 25 or 50 tests. Kit Includes positive and negative controls. 50 Test Kit includes 2 additional test sticks to run QC.
|
|||||||||||||||||||||||||
|
A color immunochromatographic assay intended for the qualitative detection of Group A Streptococcus antigen directly from throat swab specimens. Features and Benefits
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
||||||||||||||||||||||||||
 |
OSOM® Mono Test
SEKISUI DIAGNOSTICS
Detects heterophile antibodies to EBV (monocucleosis) in serum, plasma, or whole blood. Easy-to-read two color results. 100% sensitivity, 99% specificity. No age restriction. Result in five minutes or less. Room temperature storage.
|
|||||||||||||||||||||||||
|
For the qualitative detection of infectious mononucleosis heterophilic antibodies in serum, plasma or whole blood. Features and Benefits
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
||||||||||||||||||||||||||
 |
OSOM® BVBLUE® Test
SEKISUI DIAGNOSTICS
The OSOM BVBLUE Test detects elevated vaginal fluid sialidase, an enzyme produced by pathogens associated with Bacterial Vaginosis including Gardnerella, Bacteroides, Prevotella and Mobilincus. CLIA-waived objective results in 10 minutes.
|
|||||||||||||||||||||||||
|
Detects elevated vaginal fluid sialidase activity, an enzyme produced by bacterial pathogens associated with bacterial vaginosis including Gardnerella, Bacteroides, Prevotella and Mobiluncus. Features and Benefits
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
||||||||||||||||||||||||||
 |
OSOM® Strep A
SEKISUI DIAGNOSTICS
Clear, accurate results in 5 minutes or less. Easy-to-read two color results - 50 Tests - Includes 2 additional tests for external QC. Room temperature storage - Controls included in kit.
|
|||||||||||||||||||||||||
|
A color immunochromatographic assay intended for the qualitative detection of Group A Streptococcus antigen directly from throat swab specimens. Features and Benefits
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
||||||||||||||||||||||||||
 |
OSOM® H. pylori Test
SEKISUI DIAGNOSTICS
Detects IgG antibodies to H. pylori in serum, plasma or whole blood. CLIA-waived for whole blood. 95.9% sensitivity. Result in 10 minutes or less. 18 month room temperature storage.
|
|||||||||||||||||||||||||
|
For the qualitative detection of Helicobacter pylori antibodies in serum, plasma or whole blood as an aid in the diagnosis of H. pylori infection. Features and Benefits
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
||||||||||||||||||||||||||
 |
OSOM® Ultra Plus Flu A&B Test
SEKISUI DIAGNOSTICS
The OSOM® Ultra Plus Flu A & B Test by Sekisui is an in vitro rapid qualitative test that detects influenza type A and type B nucleoprotein antigens directly from nasal swab and nasopharyngeal swab specimens obtained from patients with signs and symptoms of respiratory infection.
|
|||||||||||||||||||||||||
|
OSOM® Ultra Plus Flu A&B Test Stronger Clinical Performance Takes Lateral Flow Testing To The Next Level Providing superior rapid results at the point-of-care. Fast, easy, cost effective so you can test and treat in one visit.
The OSOM®Ultra Plus Flu A & B Test is an in vitro rapid qualitative test that detects influenza type A and type B nucleoprotein antigens directly from nasal swab and nasopharyngeal swab specimens obtained from patients with signs and symptoms of respiratory infection.
*Refer to the Package Insert for additional performance claims.
SEKISUI DIAGNOSTICS
4 Hartwell Place | | Lexington | MA |
For over 35 years Sekisui Diagnostics has been committed to providing innovative medical diagnostics to physicians and laboratories. We develop, manufacture, and supply billions of tests each year to the global healthcare market. Our product lines include clinical chemistry and coagulation systems and reagents, point-of-care molecular, rapid tests and immunoassay system as well as enzymes and specialty biochemicals.

|
||||||||||||||||||||||||||
 |
OraQuick HCV
ORASURE TECHNOLOGIES, INC.
The OraQuick® HCV Rapid Antibody Test is the FIRST and ONLY FDA-approved, point of care test for the detection of HCV antibodies. Our simple, CLIA-waived platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes. The test can be completed in 3 simple steps from 5µl of fingerstick or venous whole blood with less than 2 minutes of hands on time.
|
|||||||||||||||||||||||||
|
The OraQuick® HCV Rapid Antibody Test is the FIRST and ONLY FDA-approved, point of care test for the detection of HCV antibodies. Our simple, CLIA-waived platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes. The test can be completed in 3 simple steps from 5µl of fingerstick or venous whole blood with less than 2 minutes of hands on time. It is ideal for testing programs in both clinical and non-clinical settings, as well as outreach events. Examples include urgent care, labor and delivery, laboratories and physician offices  OraQuick HCV Rapid Antibody Test The FIRST and ONLY FDA- approved, CLIA-waived test for HCV antibodies Accurate
Simple
Fast
ORASURE TECHNOLOGIES, INC.
220 East First Street | | Bethlehem | PA |
Based in Bethlehem, Pennsylvania, OraSure Technologies
develops, manufactures and markets point-of-care, oral fluid specimen collection
devices that leverage proprietary oral fluid technologies, diagnostic products,
including immunoassays and other in vitro diagnostic tests, and other medical devices.

|
||||||||||||||||||||||||||
 |
OraSure Ora Quick Advance HIV-1/2
ORASURE TECHNOLOGIES, INC.
The OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test is an FDA-approved point of care screening test for use with oral fluid, fingerstick or venous whole blood and plasma. The test delivers lab accurate results within 20 minutes with less than 2 minutes of hands on time. CDC guidelines call for routine testing in all healthcare settings to identify all HIV positive people and connect them to care.
|
|||||||||||||||||||||||||
|
The OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test is an FDA-approved point of care screening test for use with oral fluid, fingerstick or venous whole blood and plasma. The test delivers lab accurate results within 20 minutes with less than 2 minutes of hands on time. CDC guidelines call for routine testing in all healthcare settings to identify all HIV positive people and connect them to care. This simple test is ideal for testing programs in both clinical and non-clinical settings, as well as outreach events. Examples include urgent care, labor and delivery, laboratories and physician offices.  OraQuick ADVANCE HIV-1/2 Rapid Antibody Test The early HIV antibody detection test for recent infection Sensitivity
Versatility
Reliability
ORASURE TECHNOLOGIES, INC.
220 East First Street | | Bethlehem | PA |
Based in Bethlehem, Pennsylvania, OraSure Technologies
develops, manufactures and markets point-of-care, oral fluid specimen collection
devices that leverage proprietary oral fluid technologies, diagnostic products,
including immunoassays and other in vitro diagnostic tests, and other medical devices.

|
||||||||||||||||||||||||||
 |
cobas® Influenza A/B assay
ROCHE DIAGNOSTICS
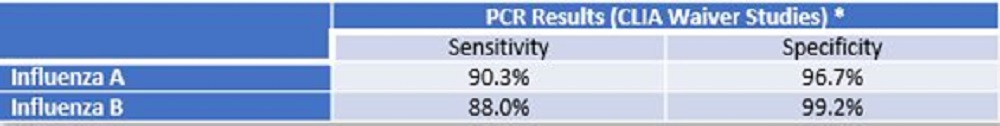
The cobas Influenza A/B assay is the first CLIA-waived, real-time PCR test to detect Influenza A and B in ~20 minutes
|
|||||||||||||||||||||||||
|
cobas® Influenza A/B assay The cobas® Influenza A/B assay is the first CLIA-waived, real-time PCR test to detect Influenza A and B in ~20 minutes. Available for use in non-traditional testing sites, including ERs, physician offices, pharmacy clinics and other urgent care settings. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
||||||||||||||||||||||||||